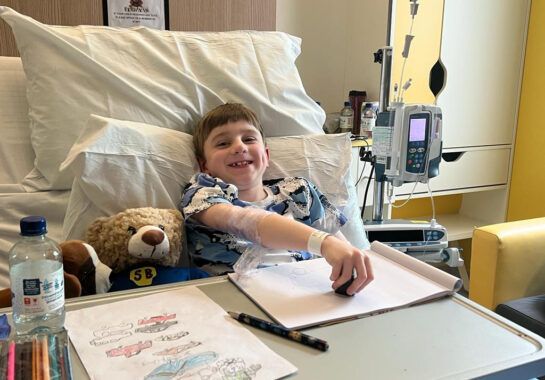
The NIHR Alder Hey Clinical Research Facility (CRF) at Alder Hey gave the Trust’s first gene therapy treatment as part of a research study for patients with Duchenne muscular dystrophy (DMD).
DMD is a progressive condition that causes increasing muscular weakness in children, including difficulty standing and walking, and can lead to heart and breathing complications later in life. There currently is no cure for the condition.
Teddy was diagnosed with the condition 2 years ago and he and his family have been through a rigorous screening programme to be eligible for the study. The treatment involved replacing the gene that is not working properly in Teddy’s cells with a healthy version.

It would not have been possible to have run such a complex, highly specialist research study without lots of help, including charities such as Joining Jack and Duchenne UK, who have funded a number of roles in DMD research at Alder Hey, and the DMD Hub who help coordinate study access across the UK.
It’s the best opportunity that he can be given, but it’s also a strong push in the right direction for the future of other children. He’s making history.”
Teddy’s parents
The NIHR Alder Hey Clinical Research Facility (CRF), recently renewed for another 5 years, specialises in undertaking complex studies of medicines at the beginning of their development, like this study. Specialist consultants, nurses, pharmacists, play specialists and more all based on the CRF have all helped deliver this research, as well as staff across the rest of the trust.
Dr Dan Hawcutt (CRF Director) said: “This study seems to have caught the imagination of the Trust, and on behalf of the CRF team I would like to thank everyone who has helped us get to this point, including Radiology, Cardiology, and ward 4C, as well as many other unsung heroes.”
Congratulations to all the staff involved for their amazing work.